This is the first article in our kakigori series. It is followed by our article on the why is kakigori ice special.

At first glance, kakigori appears to be a simple preparation. Just some ice, shaved with a machine with some added toppings. But if you’ve eaten a lot of kakigori, you’ll start to realize taste and textural differences between shop to shop. As it turns out, there’s a lot more to ice than simple frozen water. To better understand this, we’ll first have to cover the scientific concepts that underpin the freezing of water and formation of ice crystals.
The basics of a water molecule
As you’ved have learned in high school chemistry lessons, a single molecule of water is composed of two hydrogen atoms bonded to one oxygen atom. The bonds between the atoms are known as covalent bonds, formed from the sharing of electrons between the hydrogen and oxygen atoms.
The word ‘share’ is a bit of an oversimplification but for the purpose of our understanding, the sharing isn’t exactly equal. Instead, the electrons spend a bit more time at the oxygen end of the molecule, making the oxygen end slightly negative. Conversely, since the electrons are not near the hydrogen end as much, that end is slightly positive. Such a molecule is known as a polar molecule.
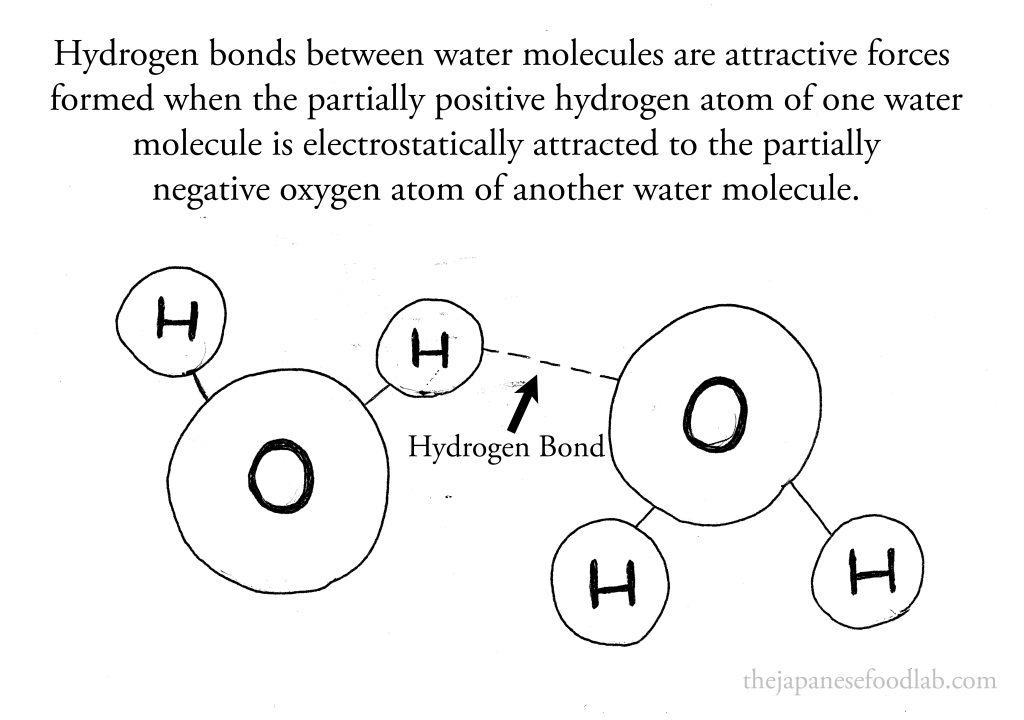
While covalent bonds are what hold the atoms in a water molecule together. What we’re more interested in are hydrogen bonds, which occur between water molecules and are weaker than covalent bonds. Hydrogen bonds occur between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of neighboring water molecules.
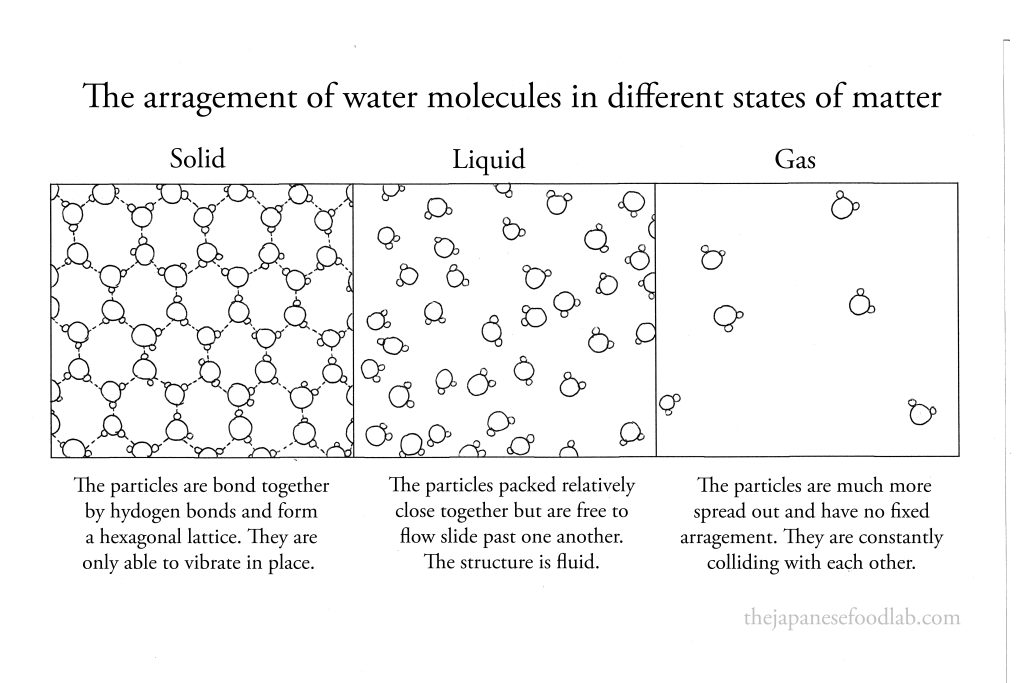
What is the arrangement of water molecules in different states of matter?
When frozen solid, water molecules are arranged in a hexagonal lattice and held in place by hydrogen bonds.
As a liquid, the water molecules are still bonded to one another but are constantly forming, breaking and reforming hydrogen bonds with neighboring water molecules.
As a gas, water molecules are moving around very quickly and constantly colliding with one another. Hence, hydrogen bonds are unable to form between molecules.
Why does water have such a high boiling point?
Relating this back to the hydrogen bonding mentioned above helps us understand the high boiling point of water. When liquid water is heated, its molecules gain kinetic energy, causing them to move more vigorously. As the temperature continues to increase, the molecules gain enough energy to break the hydrogen between molecules. When this occurs, the water molecules are able to break free from their current state, transitioning from a liquid (water) to a gas (steam) as it reaches boiling point. Because a lot of energy is needed to break the hydrogen bonds between molecules, water has a comparatively higher boiling point compared to other substances of a similar molecular weight.
What happens as water cools?
When hot liquid cools down, water molecules lose kinetic energy, causing them to move more slowly. As the temperature continues to decrease, the molecules lose their ability to overcome the attraction between the positively charged hydrogen atoms of a water molecule and the negatively charged oxygen atom of another molecule. When this occurs, hydrogen bonds begin to form between molecules and the molecules take on a more ordered arrangement. However in a liquid state, the molecules still have enough kinetic energy to cause the hydrogen bonds between molecules to constantly form, break and reforming, hence explaining why liquid water is still able to flow.
If any of the last two sections sounds familiar to you, it’s most likely because it’s the standard answer to a chemistry exam question you’d get in highschool.
What happens when water freezes
When water reaches its freezing point, the molecules have lost so much kinetic energy that they are no longer able to resist the forces of attraction between molecules and therefore can no longer break hydrogen bonds. As this occurs, they start to arrange themselves into a crystal structure. This structure takes the form of a hexagonal lattice due to the most energetically favorable arrangement of hydrogen bonds between neighboring water molecules. As the water continues to freeze, the hexagonal lattice actually causes the water molecules to have more space in between them compared to liquid water, which is why ice is less dense than liquid water.
In addition, water doesn’t just start freezing and arranging themselves randomly. Instead, this process usually occurs at nucleation points that act as sites where ice crystals begin to form within the water. Some common nucleation points in a typical freezer include:
- Impurities: Tiny particles or impurities present in the water can serve as nucleation sites. These particles might include dust, minerals, or other contaminants that initiate the formation of ice crystals.
- Surface irregularities: Any irregularities or imperfections on the surface of the container or the freezer itself can act as nucleation sites. For instance, scratches or rough patches in the container can promote the initial formation of ice crystals.
- Agitation or movement: Disturbances caused by the movement or shaking of the container can introduce nucleation sites. Agitating the water can induce the formation of ice crystals by disrupting the water’s stability.
- Temperature fluctuations: Sudden temperature changes or fluctuations within the freezer can create conditions conducive to nucleation. Variations in temperature can prompt the water to reach its freezing point, initiating ice crystal formation.
- Air bubbles: Air trapped within the water can also act as nucleation sites. Gas bubbles provide surfaces where ice crystals can start forming.
These nucleation points allow the water to transition from a liquid to a solid state by initiating the formation of ice crystals. Once the process starts at these points, it can propagate rapidly throughout the water, leading to the solidification of the entire mass of liquid into ice.
Why does water have such a high freezing point
On the topic of freezing, water’s freezing point is actually unique compared to many molecules of similar molecular weight. Most substances with a similar molecular weight to water have different freezing points. For instance, hydrogen sulfide and ammonia are gaseous at room temperature and pressure, whereas water is a liquid.
The explanation is slightly nuanced but we thought we’d cover it from a point of scientific curiosity. If we recall that molecules in liquid water are constantly breaking and forming hydrogen bonds with each other, some of these hydrogen bonds do not arrange the water molecules in a way that promotes the formation of a stable, ordered crystalline structure, and thus need to be broken for the molecules to arrange themselves into a hexagonal lattice. This extra energy required to break the hydrogen bonds contributes to the higher freezing point of water compared to many other liquids.
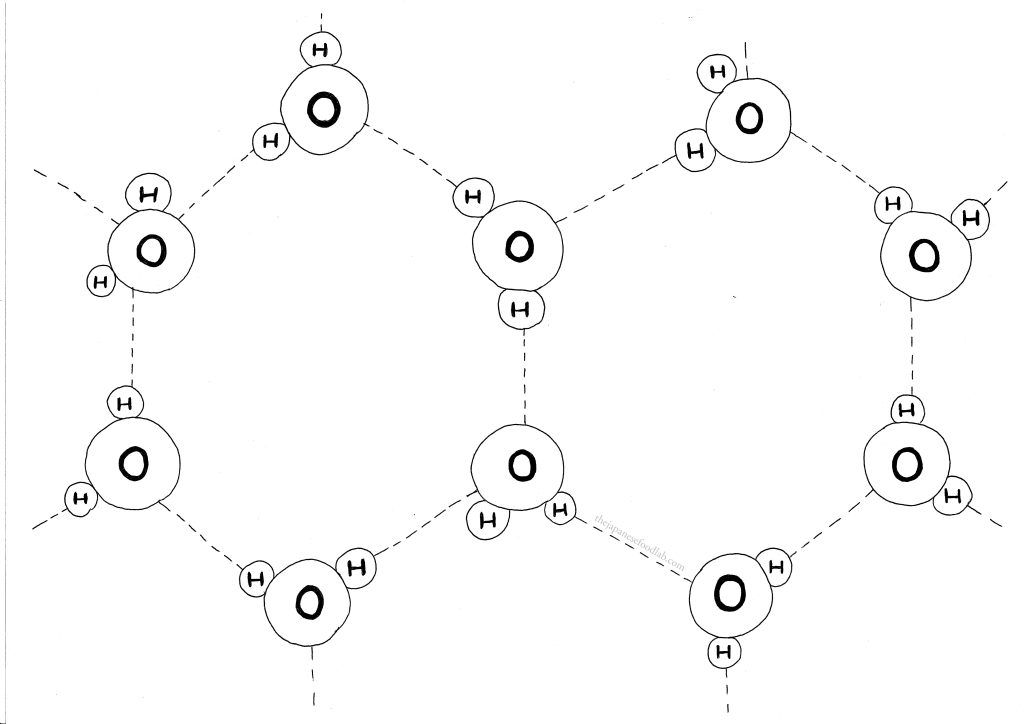
What are ice crystals?
As water continues to freeze, the hexagonal lattice continues to grow larger and larger as more and more water molecules form hydrogen bonds with it. At this point, this hexagonal lattice of water molecules is usually referred to as ice crystals though “ordered crystalline structure of ice” usually refers to the broader, macroscopic arrangement of water molecules in the solid state, while “ice crystals” describe the individual, microscopic units that collectively compose ice. A solid block of ice consists of many ice crystals packed together, each contributing to the overall structure and stability of the ice.
What factors affect the formation of ice crystals?
| Factor | Effect on ice crystal formation |
| Temperature | Lower temperature results in smaller ice crystals |
| Pressure | Higher pressure results in smaller ice crystals |
| Agitation | Greater agitation results in smaller ice crystals |
| Purity | Lower purity results in smaller ice crystals |
While ice crystals will continue to form so long as there is liquid water present below freezing point, the rate at which this occurs is influenced by several factors.
As a rule of thumb, the faster water freezes, the smaller the ice crystals whilst the slower the freezing the larger the ice crystals. This is because when water freezes quickly there’s less time for the water molecules to organize themselves into larger crystal structures. Instead, small ice crystals form due to the rapid decrease in temperature, trapping the molecules in a more compact arrangement before they have a chance to grow into larger structures.
Conversely, slower freezing allows more time for the water molecules to align and form larger ice crystals. When the freezing process is gradual, molecules have more time to arrange themselves into more extended structures, resulting in larger ice crystals.

This is why commercial factories use blast freezer or other rapid freezing technologies to increase the rate of freezing in order to preserve food as the growth of large ice crystals will destroy the texture of food, making it mushy when defrosted. The same logic goes for sashimi. It’s impossible to freeze fish for sashimi in a home freezer as the rate of freezing is too low, resulting in the fish losing its texture when defrosted. However, a specialty sashimi freezer can get the temperature of the fish low enough to kill parasites before any large ice crystals form, preserving its texture when defrosted.
Temperature
The easiest way to increase the rate of freezing is to simply lower the temperature as much as possible. Water put in a -20°C freezer will freeze much faster than in a freezer at -2°C.
Pressure
Whilst not as important when making kakigori, at a constant temperature, increasing pressure decreases the freezing point of water. This happens because higher pressure prevents molecules from moving as freely, thus impeding their ability to form the ordered structure necessary for freezing and forming larger ice crystals.
Water purity
Impure water typically has a lower freezing point compared to pure water. When impurities such as salt or minerals are dissolved in water, they disrupt the formation of ice crystals. This interference makes it more difficult for the water molecules to arrange themselves into the structured pattern needed for freezing. Consequently, impure water will generally require colder temperatures to freeze compared to pure water. Furthermore, water containing impurities or foreign particles might generate smaller crystals as these particles can serve as nucleation sites for crystal formation. Conversely, pure water tends to produce larger ice crystals due to the absence of impurities that can act as nucleation points.
Agitation
When water is agitated at a constant temperature, the movement disrupts the formation of large ice crystals by constantly breaking and preventing them from growing. The constant movement prevents the water molecules from settling into a stable position necessary for the formation of large, structured ice crystals. Instead, smaller crystals form as the agitation continues, effectively slowing down the freezing process by impeding the growth of larger ice crystals.
While it may seem that we have just covered a lot of science that has very little to do with kakigori, all the information in this article lays the foundational knowledge required to perfect the ice used. To learn more, read our article on the science of kakigori.
*Hydrogen bonds are a type of weak chemical bond that occurs when a hydrogen atom, which is covalently bonded to a more electronegative atom such as oxygen, nitrogen, or fluorine, experiences an attractive force towards another electronegative atom in a neighboring molecule. In the case of water molecules, each hydrogen atom is slightly positive due to the unequal sharing of electrons with the more electronegative oxygen atom, which becomes slightly negative. This polarity allows the slightly positive hydrogen atoms of one water molecule to be attracted to the slightly negative oxygen atoms of adjacent water molecules. These interactions, known as hydrogen bonds, are significantly weaker than covalent bonds but are crucial for the unique properties of water, such as its high boiling point, surface tension, and ability to dissolve various substances. Hydrogen bonds play a fundamental role in the structure and function of many biological molecules, including DNA and proteins, by stabilizing their three-dimensional structures.