Having covered the foundational science of ice formation, in this article, we’ll take a deep dive into how the way we freeze ice affects the taste and texture of kakigori.

What does “good” kakigori taste like?
If you’ve only ever had the shaved ice at festivals, you’ll find that they have a crunchy, almost sharp texture, as well as a very cold mouthfeel, so much so that if you left it for a while, the bits of shaved ice would start to fuse into one another and form large chunks of ice. It’s perfect for its purpose, cheap and quick to make and buy, easy to eat and most importantly, cools you down during a hot summer day.
However, modern kakigori specialists in Japan are pushing shaved ice into a totally different territory. There, the shaved ice is thin yet fluffy and melts quickly when you put it in your mouth, melding together with the flavors of all the toppings added.
Why is ice crystal formation important when making kakigori?
If you were to closely observe the block of ice being shaved at a kakigori restaurant, you’ll notice that the block of ice is clear and transparent. Whilst a seemingly benign detail, it actually hints towards everything you need to know about kakigori ice.
Large vs small ice crystals
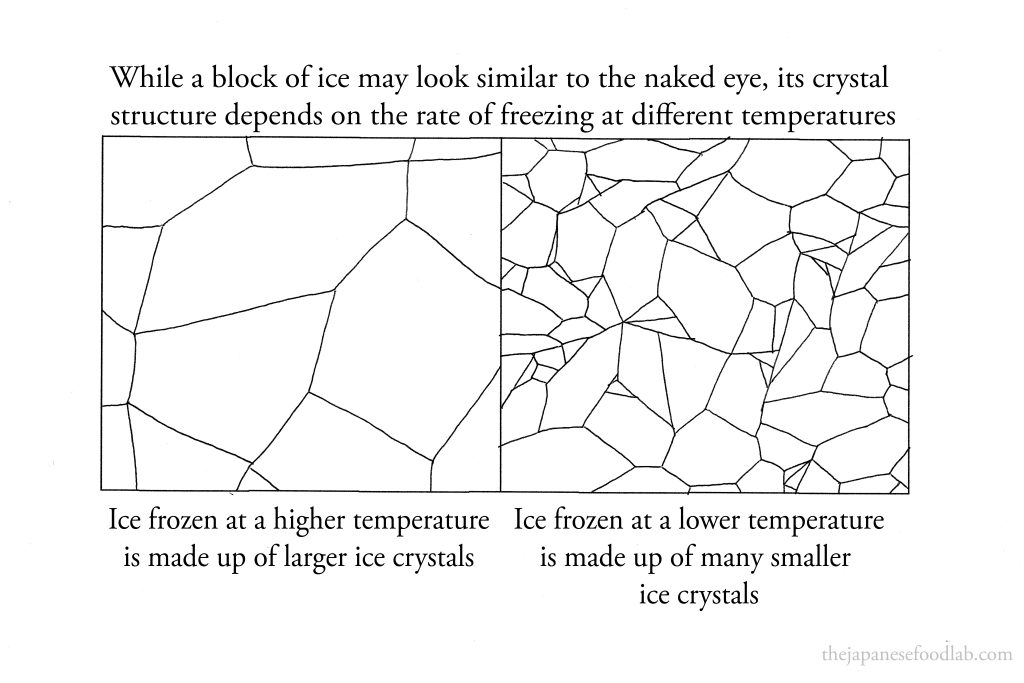
A block of ice is actually made out of many ice crystals fused together. However, the size of the ice crystals that make up the ice can vary in size. Typically, clear ice contains larger water crystals compared to cloudy or opaque ice. The clarity or transparency of ice is often associated with the size and uniformity of its ice crystals.
Clear ice forms when water freezes slowly and uniformly. During slow freezing, water molecules have more time to arrange themselves into larger, well-organized crystals. These larger crystals are more likely to exclude impurities and trapped air bubbles, resulting in clearer ice.
On the other hand, cloudy or opaque ice forms when freezing occurs rapidly or inconsistently. Rapid freezing doesn’t allow enough time for water molecules to form large, ordered crystals. Instead, small crystals form quickly, trapping air bubbles and impurities within the ice, causing cloudiness.
The presence of air bubbles, impurities, or irregular crystal formations in cloudy ice scatters light as it passes through the ice, resulting in a cloudy appearance. This scattering of light creates the opaque or cloudy appearance of the ice, which is due to the irregularities in crystal size and structure.
Why does clear ice make the best kakigori?
This all comes down to the size of ice crystals that make up the block of ice. To understand this, we first want to consider the difference between “breaking” ice and “shaving” ice.
The difference between breaking and shaving ice
Breaking involves applying force to a material in a way that causes it to fracture or separate into pieces. In terms of ice, you can break a piece of ice by separating the ice crystals that make up the ice. This can be done by hitting the ice with a mallet, using an ice pick, or applying pressure to break it into irregular shapes or smaller pieces. It typically results in irregular or jagged edges and uneven surfaces on the fractured pieces. Because you’re simply breaking apart the ice crystals from each other, you won’t be obtaining a smooth surface.
Conversely consider shaving as removing thin layers or slices from the surface of a material to shape or refine it. This method is more precise and controlled compared to breaking. In terms of ice, this means actually cutting through the ice crystals to remove a thin layer instead of simply breaking them apart and without causing it to fracture. It’s often used to create smoother and more even surfaces.
Making kakigori from clear vs cloudy ice
In general, a block of cloudy ice with smaller ice crystals is more prone to breaking but will not shave as well compared to clear ice with larger ice crystals.
Smaller ice crystals mean that the overall structure of the ice might be less cohesive due to the higher number of boundaries or interfaces between these smaller crystals. When force is applied, these boundaries between smaller crystals can act as points of weakness, making the ice more susceptible to breaking or fracturing along these lines. When you attempt to shave it, smaller crystals, with more irregularities and boundaries between them, will create a rougher surface when shaved due to the interrupted and less uniform structure. This will not only create kakigori with an undesirable rough mouthfeel but will also not yield a melt in your mouth texture as it would be impossible to shave a thin layer of ice.
Conversely, clear ice, characterized by larger ice crystals with fewer boundaries, tends to be more cohesive and less prone to breaking. Its more uniform structure means that it can withstand force more effectively, resulting in fewer fractures or breaks when pressure is applied. Moreover, when shaved, larger crystals of clear ice can produce finer and more consistent snow-like textures, enhancing the overall experience of the shaved ice dessert. The smoother surface obtained from clear ice allows for thin and delicate shavings, contributing to a velvety, melt-in-the-mouth sensation that’s highly sought after in kakigori.
This comparison is highly unintuitive. Who would have thought that ice that is harder to break is actually easier to shave, and that the reverse is also true?
In conclusion, to produce the best kakigori, we want clear ice made from large ice crystals. To learn how, read our next article on the science of freezing ice.