In addition to having better shaving properties as elaborated in our article on kakigori ice, clear ice also melts slower as it lacks impurities, allowing you to enjoy your kakigori for longer periods of time. But how do you make clear ice at home and what is the science that underpins this?

What are the variables when making clear ice for kakigori?
To produce clear ice, several variables need to be controlled during the freezing process. These include:
- Temperature
- Water purity
- Directional freezing
You’ll notice that all of these are the same factors that affect the rate of freezing and formation of ice crystals. This makes sense as clear ice is formed during a slow rate of freezing that leads to a formation of large ice crystals. Here instead, we’ll focus on how we can control these variables.
Temperature
To make clear ice, your best bet is to set the temperature as close to freezing point as possible. In a perfect world, you’d always go with 0°C but unfortunately a large amount of water could take several days in a fridge set at this temperature to fully freeze, and that’s assuming your fridge is fully accurate. More realistically would be to experiment with a temperature between -1°C and -10°C.
A higher temperature allows a more gradual rate of freezing, which in turn gives molecules more time to arrange themselves into a hexagonal crystalline structure. More importantly, more water molecules can also bind to the lattice and grow the size of the crystal. At a lower temperature, another crystal would most likely have started to form instead of the molecules attaching to pre-existing crystals, giving rise to many smaller ice crystals instead of one large crystal.
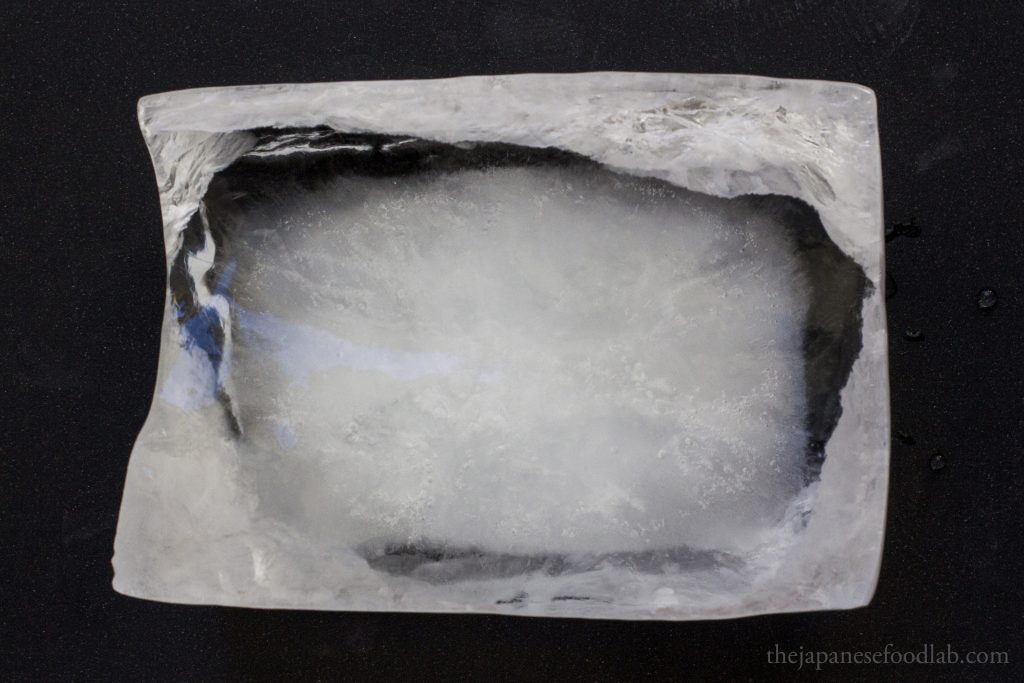
Directional freezing
Industrial operations that specialize in producing clear ice for commercial use have special equipment to only freeze water from one direction. If water is slowly frozen from a single direction, such as from the top down or from one side, the ice crystals start growing from that direction as the molecules arrange themselves into a crystalline structure. Because the crystalline structure is only forming from one direction, any impurities or air bubbles in the water are pushed away from the direction of freezing.
Why does this occur? Though slightly unintuitive, when water molecules freeze into ice, they release heat (known as latent heat of fusion). This heat warms the immediate surrounding water slightly, creating a zone of slightly higher temperature in the vicinity of the freezing front. This zone of slightly warmer water contains more impurities and air bubbles that haven’t yet been incorporated into the ice crystals. As the ice continues to form in a directional manner, driven by the release of latent heat, this slightly warmer and impurity-laden water is pushed away from the freezing front. The direction of the heat transfer and the movement of this slightly warmer water help in displacing impurities and air bubbles towards areas away from where the clearer ice is forming.
Using a water cooler
While it would be impossible to obtain a directional freezer at home, luckily for us the trick of putting a cooler box into a freezer to slow the rate of freezing also helps us solve this problem. By simply leaving the lid off the cooler box, the cold air of the freezer only comes into contact with water at the opening of the cooler, mimicking directional freezing.
While it might seem weird to put a cooler box into a freezer, unfortunately most home freezers do not allow you to precisely control the temperature. In this case, the use of an insulating container or cooler is useful to slow down the rate of freezing by insulating the water from the cold temperature.

Water purity
While directional freezing can help push away impurities in the ice, it’s much easier to make clear ice by starting with water with a low level of impurities. Commercial ice makers will run their water through several filters and even multiple rounds of reverse osmosis. If for some reason you already have such equipment in your home or restaurant, say you’re a coffee enthusiast or fish aquarist, then you’re ready to go. If not, you’ll either want to purchase drinkable distilled water from a store, or try to purchase softer water. If this is too much effort or not cost efficient where you live, simply go with the purest water you can obtain, be it filtered water or boiled water. You won’t achieve the same results but it’ll be clear enough if you combine it with using a cooler.
So why does water purity matter? Impure water also often contains dissolved gasses like oxygen and nitrogen. During freezing, these gasses can get trapped within the ice, forming bubbles. These bubbles scatter light as it passes through the ice, causing cloudiness or opacity. Minerals present in water, such as calcium, magnesium, or salts, can also affect ice clarity. They might not incorporate uniformly into the ice crystal lattice, leading to irregularities and cloudiness. Additionally, some minerals might remain as residues when the water freezes, contributing to a less clear appearance.
The minerals and gasses in the water can also act as nucleation points, initiating the formation of ice crystals. When water contains impurities like dust, minerals, or other particles, these substances can serve as sites where ice crystals start to form. This results in a less uniform crystalline structure made from many small ice crystals and increases the chances of cloudy or opaque ice. Conversely, pure water will freeze clear as the lack of nucleation points will mean that water molecules will simply bind to any pre-existing crystal structure and grow into large crystals rather than form new small ice crystals.
The trick of using boiling water
One trick that is sometimes used to achieve clearer ice is to place hot water that has just been boiled straight into the freezer. Before you do this, I must advise that this quickly raises the temperature of the freezer and so if you have other frozen food inside it will most likely thaw and spoil. Only do this if you have an empty freezer or one with a very powerful compressor.
The reason why this works is that the solubility of gasses in water is inversely proportional to temperature. This means that hot water will contain less dissolved gasses compared to cold water and thus less impurities. Just like how water molecules are attracted to one another in liquid water, the dissolved gasses in the liquid are also attracted to water molecules in the liquid.
When water is heated, its molecules gain kinetic energy and start moving around faster. This increased kinetic energy disrupts the bonds between water molecules and gas molecules, making it easier for the gasses to escape from the water into the surrounding air, thus explaining the reduced solubility.

If you’re interested in learning more about this, you can look up Henry’s Law that relates the concentration of a gas dissolved in a liquid to the partial pressure of that gas above the liquid at a specific temperature. Mathematically, The constant that describes the solubility of a gas at a given temperature is called the Henry’s Law constant.
Henry’s Law constant changes with temperature due to the alteration in the solubility of gasses in a liquid. At higher temperatures, the solubility of gasses in a liquid generally decreases. This decrease in solubility means that less gas can dissolve in the liquid at higher temperatures compared to lower temperatures. Therefore, as the temperature increases, the solubility of gasses in the liquid decreases, resulting in a change in the Henry’s Law constant to maintain the relationship between gas concentration and partial pressure.
However, boiling water is not completely effective in removing all types of impurities. It can reduce the concentration of some impurities to a certain extent, especially those that have a lower boiling point than water or are volatile. But substances with higher boiling points or those that do not easily vaporize might remain in the water even after boiling.
For instance, minerals, heavy metals, or non-volatile contaminants generally won’t be removed through boiling. To eliminate these impurities, additional purification methods like filtration, distillation, or reverse osmosis are often necessary.
What’s next
Now that we’ve learned how different variables affect the growth of large ice crystals that are crucial to making clear ice, we can see the step by step process in action.
Hello. I was reading through the kakigori articles and it appears to get cut off after a few pages. The last page available ends with the following:
“Now that we’ve learned how different variables affect the growth of large ice crystals that are crucial to making clear ice, we can see the step by step process in action.”
After pushing “Next”, it takes me to a different article.
Is there any way to correct this or are you able to send me a PDF copy? I have been very much enjoying the content. I love how thorough everything is and how much detail you put into it. I have been considering adding kakigori to my shop or even opening a shop of its own, so this has been very helpful in my research. Thank you!
Unfortunately, no the articles are not written yet. All the information, testing, and research that has been done to the level of detail that you enjoyed requires several months of work. Just to give you an example, the 3 articles that you have just finished reading took 10 weeks to finish up.
We already do our best to run this website as best as possible. So please be patient.