
*The bulk explanation of the chemistry behind what is going on is at the end of the article.
The two main coagulants used in Japan to make tofu are Nigari (にがり) and gypsum salts (石膏塩), both which can be extracted from seawater using the household technique below. First, let’s have a look at seawater. Depending on where you live, seawater typically consists of around 3 to 3.5% of salt. This means that in 1 litre of seawater, you have around 30 to 35g of salt. But what do we mean here by salt? The most common salt we find in our cupboards consists almost 100% of sodium chloride (NACl) with maybe a small amount of trace minerals in it. This is because salt production factories put in a lot of effort to sterilize and filter their product before it reaches our supermarket shelves. You can read up more about it but by google-ing salt processing and purification and how bittern is removed from salt. However, these trace minerals are important as they are where our fancy salts come in. For example, most tests on the famous pink Himalayan salt done have shown that it consists of around 99% sodium chloride as well, with the remaining 1% of magnesium, calcium and potassium giving it its famous pink colour. This actually means that the extremely expensive price you pay for pink salt is just marketing as the product is 99% similar. So why is this relevant to us? This is because we’re trying to extract the small amount of trace minerals in the form of Nigari (magnesium chloride, MgCl2) and gypsum salt (calcium sulphate, CaSO4) present in seawater using what we have at home.
Why does the salt industry go to such great lengths to purify their salt?
The main concern here with using any old seawater is impurities present in seawater. If you think about all the rubbish and pollutants in the ocean, you don’t want to be putting that into your food. And it’s not the big particles that we can see that we’re worried about, but the small micro-particles that are the most dangerous. If you’re talking about bits of rubbish, human skin or dirt in seawater, that’s easy enough to filter out. It’s the small traces of mercury and arsenic etc that will be the problem. Then there’s the even worse problem of radioactive water used to cool nuclear reactions being flushed into oceans, especially during a nuclear reactor crisis, which can contaminate seawater, which is why I’ve read of some factories that even test their nigari for radioactivity near Fukushima. Lastly, there’s also the danger of contamination from fungal or bacterial microorganisms in the water that needs to be sterilized first. Whilst most of these microorganisms won’t be able to withstand the osmotic pressure from the salt when it crystallizes, the toxins produced by them may sometimes remain. Another interesting fact to point out may be that gypsum (calcium sulphate, CaSO4) is actually correlated with the formation of kidney stones. So whilst gypsum salt is used to coagulate tofu in smaller concentrations, higher concentrations of it is probably not good for you. Though a diet high in tofu poses you no threat as long as you drink enough water.

What filtration processes occur industrially to clean the seawater?
Other than the typical water filtration and UV light filtration that you get, you also have automatic backwash filters, semipermeable membrane filters etc, most we aren’t interested in for the sake of this article. All you need to know is that they clean the water of dangerous impurities. The impurities in large salt ponds can be removed in another way however. Let me explain: one way of extracting salt from seawater however is to pump seawater into shallow inland ponds that then are then left to evaporate in the sun. As the water evaporates more and more, the sea water becomes more and more concentrated until the salt crystallizes. Whilst the concentration at which different salts crystallize varies according to temperature and other factors, as a vast generalisation, the sodium chloride in these ponds tends to crystallize first, leaving behind a liquid that contains the remaining impurities like magnesium, calcium and sulphur. This liquid is called bittern and actually translates to nigari in Japanese and can be used to coagulate tofu! Of course the bittern in these salt lakes are full of other impurities which is why they are typically drained (or drawn off) from the ponds to clean the salt. The bittern from these salt lakes are much dirtier compared to the nigari that you can buy off the shelf in a japanese supermarket which is much more filtered and purified.
Is it possible to make homemade nigari from scratch?
*The following section is science heavy and understanding it is not required to follow this recipe.
When first looking at this, my immediate thoughts were on how it would be possible to precipitate out magnesium chloride from a solution that contained a variety of ions (i.e seawater), which seemed like a very complicated thing to do. This led me to the Dow process, which is used in the industry to achieve this. Patented by Herbert Henry Dow in 1891 to commercially generate bromine, the same chemical reaction can be used to extract magnesium chloride (nigari). First, seawater is slurried with calcium dolomite (CaMg(CO3)2) which causes the magnesium to precipitate out as magnesium hydroxide which is an alkaline. Then though a simple acid-alkaline neutralisation with hydrochloric acid, you get magnesium chloride and water. The reaction is shown below:
Mg2++Ca(OH)2→Mg(OH)2+Ca2+
Mg(OH)2+2HCI→MgCI2+2H2O
This produces a magnesium chloride solution that can be dehydrated to get a powder. The process is far too complicated to carry out at home of course, plus it produces a pure magnesium chloride solution. Whilst this can be used to coagulate tofu, part of what makes artisanal Japanese nigari special is the fact that it’s magnesium chloride mixed with small amounts of other compounds from seawater.
It is, however, still possible to extract nigari from seawater at home without all the fancy filtration equipment and chemicals, so long as some care is taken. The following section elaborates on this.

How to make homemade nigari?
There seems to be a technique that some people in Japan use at home that goes on the idea that when seawater is reduced to 1/10th it’s volume, calcium sulphate (CaSO4) or gypsum salt will precipitate out, allowing you to filter it out before sodium chloride (NaCl) or table salt crystallizes. Once the calcium sulphate is removed, you can then continue to evaporate the water until the sodium chloride crystallizes out, leaving the remaining bittern or nigari which you can strain out to make tofu. The instructions for doing this follows below. If you want my take on the scientific explanation of what is going on here, scroll to the bottom of the article.
Firstly, the source of seawater you choose is important. Ideally you’d want seawater that is as pure and unpolluted as possible. Whilst you might think that seawater from a holiday beach might be quite dirty due to all the people swimming in it, your main concern isn’t the dead human skin and rubbish in the sea that you can filter out, it is the more deadline unseen impurities like mercury and arsenic which are problem. So it’s much more important to know that the seawater you’re taking is far away from any factory pollutants rather than further away from tourists I think. Some people who want to make their own nigari but do not live near the sea actually buy seawater online which can be costly so I don’t recommend it, but can be done if you really want to.

- Obtain seawater from the ocean. You’d want to pick as clean and unpolluted seawater as possible when it comes to this step. I recommend using at least 5 litres to get a meaningful yield.
- Filter all the seawater twice through a coffee strainer to remove any sand and large impurities.
- Measure the volume of seawater you have. This step is important as we intend to reduce the water to 1/10th it’s volume or mass. So you can do this with a weighing scale or even more primitively, submerge a chopstick or any stick into your pot and mark where the water level is, and use a ruler to make where the 1/10th mark is to measure when the water has reduced to that level.
- Boil the water on high heat until the water is at 1/10th its volume. At this point, the water should be slightly cloudy white, with a suspension of white particles in it. These white particles are actually the calcium sulphate (gypsum salt) that has precipitated out of the water that can be used to make nigari.
- Strain out the gypsum salt using filter paper (coffee filter paper should work) and save for use later. The yield of gypsum salt from 1 or 2 litres of saltwater might be too small to coagulate tofu with, so a larger amount of salt water will be needed.
- Place the strained liquid back into a saucepan and continue to boil on medium heat.
- As the water continues to evaporate, you should see small crystals of table salt (sodium chloride) start to appear. At this point, you’d want to slowly start to lower the heat as you do not want to scorch or burn your salt.
- Continue evaporating the mixture until the sea salt has the texture of wet sand. At this point your stove should be already on low heat.
- Continue evaporating the sea salt until it has a texture similar to sorbet.
- Remove from heat and strain through filter paper. The resulting strained liquid contains magnesium chloride (nigari) which you can use to make tofu. It also contains small amounts of other ions such as potassium etc.
- Because the mixture will be quite dry, it will take some time for the nigari to drip out from the sea salt. If no water drips out from the sea salt, you’ve gone too far evaporating the sea salt and the magnesium chloride (nigari) would have crystalised and mixed with all the sodium chloride (table salt)
- To reverse this, dissolve the mixture back into water and continue evaporating again.
Several caveats do need to be mentioned however. The yield from this recipe is not massive, and therefore you’d need to evaporate quite a lot of seawater to get a meaningful yield of nigari, which can be quite energy intensive. In addition to that, the concentration of the nigari you get depends on the seawater you have and so the amount that you’d need to add to soy milk to get your tofu coagulated to just the right consistency will vary from ocean to ocean. One good thing however, is that water from the same sea will always yield nigari of the same concentration provided you evaporate the water by the same amount each time (which you can do by measuring the percentage weight loss).
Adding nigari to rice to improve rice texture
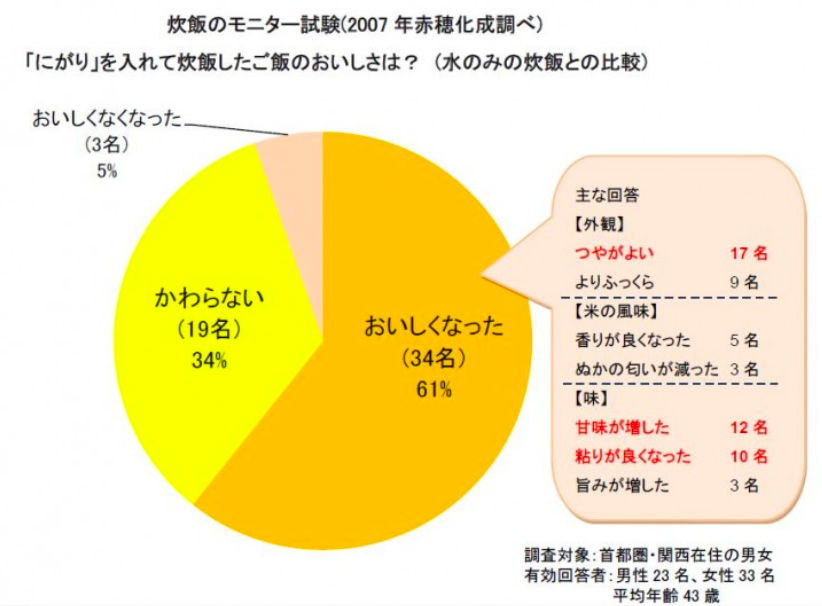
There exists a saying among chefs in Japan that you can add 3 drops of nigari to 3 cups of uncooked rice to dramatically improve the sweetness and fluffiness of rice (in the same way that people say adding salt to oranges or grapefruits makes it sweeter). This appears to have originated from a press release from the company Ako Kasei (赤穂化成株式会社) in the year 2007 claiming that in their study of cooking rice with an without nigari, the majority of people claimed that adding nigari to rice make it much more delicious. There are a few things I would like to point out from this study. Firstly, Ako Kasei is a company that produces nigari, so there does exist a conflict of interest in this study. And secondly, this study was only carried out on 56 participants (which is a respectable number) of which only 61% said the rice tasted better with nigari. I’m not sure if the participants in the study were blind to the conditions, but even then, 61% is rather close to 50% even though it’s a majority, and 50% means it’s basically down to luck whether or not the person found the rice more delicious or not. The reasoning that Ako Kasei gave behind why it made rice taste better is because the magnesium from the nigari binds to the cell wall of the rice, preventing it from rupturing and thus allowing it to retain its texture.
So does this work? Honestly if there is a difference it’s barely noticeable and the possible downsides are much worse. Because it’s hard to determine the concentration of nigari, whether store bought or self-extracted, there is a danger of adding too much nigari to the rice, and as nigari is bittern, it will cause your rice to have a bitter off taste. But that’s just my two cents on the topic.

The science behind making nigari at home. (For all the nerds out there)
When I was looking into this I kept thinking about the chemistry going on behind this household recipe. Why does calcium sulphate precipitate out of the solution when the seawater is reduced by 1/10th of its initial volume? Why does magnesium chloride crystallize out of the solution before sodium chloride? What does the solubility of a solution depend on? Doesn’t it depend on concentration and temperature? Or does the ion charges matter too. Especially when it came to sea water that had a whole host of different ions in it. My first impression was to go straight to salting out theory where the solubility of certain molecules can be reduced at very high ionic strengths. This is because the high ionic strength of the solution would cause the molecules hydrophilic and hydrophobic groups to rearrange themselves and thus precipitate out. This led on to read about Franz Hofmeister and the Hoffmeister series which organised anions and cations based on their ability to salt out (precipitate) other molecules. If I’m not mistaken, the chemical properties that place the ions in the order found in Hoffmeister’s series isn’t completely clear. For this particular case though, I thought it explained the nigari extraction perfectly, because according to the series, sodium ions have a stronger to precipitate other proteinaceous solutes and thus can precipitate out calcium sulphate. Can you spot the mistake here? Salting out theory is only applicable to large biomolecules such as DNA and proteins, not salts and minerals. I kinda felt ashamed I didn’t notice this earlier but it’s better to admit your mistakes and move on.
If it wasn’t this, then what was it? I then turned to investigate the world of evaporites, specifically marine evaporites which are water-soluble sediments or deposits that settle in an ocean after some evaporation has occurred (non-marine evaporites are ones that occur outside the ocean such as in lakes and rivers). To explain further, as ocean water evaporates, different minerals start to precipitate out as the solution becomes more and more concentrated. In fact, the order in which minerals precipitate out in sea water was recorded all the way back in 1849 by an Italian chemist known as J. Usiglio! The order of precipitation is as follows:
- Firstly, Calcite (CaCO3) and dolomite (CaMg(CO3)2),
- followed by Gypsum (CaSO4 • 2H2O) and anhydrite (CaSO4),
- followed by Halite (NaCl),
- And lastly, Potassium and magnesium salts.
Does this sound familiar! It’s actually the exact order that our home method for extracting nigari follows (excluding the fact that our seawater does not contain calcite or dolomite).
The interesting thing here is that people tend not to talk about evaporites and their ability to make tofu, but in terms of their other uses. For example, (calcium sulphate, CaSO4) is actually CaSO4.2H2O which is calcium sulphate with 2 water of crystallisation. However, when heating in a kiln, gypsum actually becomes plaster of paris, the stuff that is used in sculptures and castings. This is because when you heat gypsum, it loses some of its water as steam in the equation below:
CaSO4.2H2O → CaSO4.½H2O + 3/2H2O
The resulting CaSO4.½H2O is actually plaster of paris, which then hardens with the addition of water because it reverts back to gypsum!
So.. basically don’t make your own Nigari at home.
That’s really the best takeaway from this isn’t it?
Fascinating article. Thank you.
Thank you very much for this article and generally for all your work.
In this article you explained the extraction of nigari from seawater.
You have to get rid of the water to keeping what make the sea salty. Chemistry and gravity does the rest.
In the Book of Tofu, at the chapter devoted to Traditional Farmhouse Tofu, W.Shurtleff & A.Aoyagi mention another technique used by communities leaving far from the sea, and therefore unable to use seawater.
Those communities extracted nigari from unrefined salt.
Unrefined salt was stored in a porous bag, ambiant humidity made the bag leach out a liquid: nigari.
Farmers where using nigari to coagulate soy milk, and the refined salt left in the bag was used for shoyu and miso.
Thank you. I have made tofu with lemon and vinegar . I have yet to make tofu with nigari. The science is impressive. I wonder how it was discovered?